

 |
|
|
 |
||
| |
||
| |
|
|
| |
||
|
|
|||
 |
LEESA M. BARONE, PhD Genzyme Tissue Repair, Cambridge, Massachusetts Abstract It is generally agreed that damage involving the articular cartilage surface and confined to the cartilage undergoes little restoration. Cartilage has little intrinsic ability to heal itself. The disability and pain that result from damage to articular cartilage have stimulated the serch for ways of facilitating cartilage repair. For repaired or regenerated cartilage to perform satisfactorily as a joint tissue, it must restore normal pain-free motion of the synovial joint. Therefore, the repaired tissue must have the structure, compsition, mechanical properties, and durability similar to natural articular cartilage. A number of methods for promoting cartilage repair for chondral defects have been explored. These include debridement and lavage, subchondrial bone drilling, microfracture, abrasion arthroplasty, high tibial osteotomy, periosteal/perichondrial grafting, and mesenchymal stem cell implantation. However, with these procedures, the resulting repaired tissue is inferior to the original articular cartilage. Brittberg et al. reported a method for repairing deep cartilage defects in the femorotibial articular surface of the knee joint in humans.1 Cultured autologous chondrocytes, cells isolated from an individual's own cartilage, can be expanded in vitro and returned to the damaged site for repair of their damaged cartilage. This remarkable process is characterized by modulation of gene expression during proliferation and subsequent re-differentiation of cultured chondrocytes. This report will provide biological and molecular evidence that human chondrocytes isolated from articular cartilage and expanded in monolayer culture, retain their ability to re-express cartilage-specific phenotypic markers when inducedto differentiate. Introduction Damage to articular cartilage is an exceedingly common problem affecting
the joints of millions of people. This is major problem considering the
poor regenerative capacity of adult articular cartilage. Injuries of the
articular cartilage that do not penetrate the subchondral bone do not
heal and eventually progress to the degeneration of the articular surface. Articular cartilage (also known as hyaline cartilage) is a thin layer that covers the ends of bones in movable joints. This thin layer of tough, opaque tissue ranges from about 1 mm to 5 mm in thickness in the human knee. It permits practically frictionless motion of the bones forming the joint and is capable of absorbing load forces that are in the range of five times the body weight. While cartilage should last a lifetime, the constant wear and occasional trauma it receives can result in the degenerative process leading to osteoarthritis. This is due to the fact that articular cartilage has a poor intrinsic capacity for repair. Articular cartilage is neither innervated nor vascularized. It receives its nutrient requirements from the synovial fluid, which bathes the articular surface, or from the underlying subchondral bone. Articular cartilage is a complex, highly organized tissue (Fig. 1). The cellular component of cartilage is the chondrocyte. The chondrocytes form 1% or less of the tissue volume, however, they are the only living element in the tissue. The chondrocytes are encased within the articular cartilage matrix, which they have produced during development. The matrix consists of collagens and proteoglycan aggregates (i.e., aggrecan). Articular cartilage contains a number of different collagens: types II, XI, and IX (Fig. 2). Type II collagen is the main building block of the fibril; it provides the basic architectural structure of the cartilage. These fibers are extremely strong and have a great capacity for resisting stress. Type XI collagen is integrated throughout type II collagen and serves as a core to the fibrils. Type IX collagen is linked to type II collagen through specific covalent bonds and appears to be an intermediate between the collagen fibers and the proteoglycans. Collagen comprises about 65% of the dry weight of cartilage. The collagens provide cartilage with its tensile and biomechanical strength. An individual proteoglycan molecule consists of a central protein core to which are attached many negatively charged sulfated glycosaminoglycan (GAG) side chains like chondroitin sulfate and keratan sulfate (Fig. 3). A proteoglycan aggregate is composed of many such molecules attached to a long hyaluronic acid chain. This interaction is stabilized by link protein. Since the GAG chains are negatively charged, water molecules become trapped, forcing the proteoglycan aggregates to become distended with the water molecules. Water can account for 60-80% of the total cartilage weight. The proteoglycans and collagen types can be used as phenotypic markers for articular cartilage. The properties of articular cartilage can, therefore, be accounted fo by the collagen, which provides structure and strength, and by the proteoglycans engorged with water, which provide resistance to compression. |
|||
Figure 1. Schematic of Articular Cartilage. The cartilage matrix consists of long collagen fibers (blue-green) which surround and constrain the hydrated proteoglycan aggregate (yellow); a chondrocyte lies in the background |
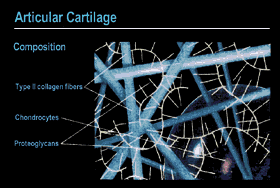 |
Figure 2. Schematic of a Collagen Fibril of Articular Cartilage. The main collagen in cartilage is type II collagen. These fibers provide the basic architectural structure of cartilage. They are extremely strong and have a great capacity for resisting stress. Type IX and XI collagens are minor collagens. |
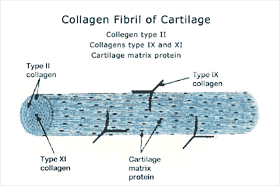 |
Figure 3. Schematic of a Proteoglycan Aggregate of Articular Cartilage. An individual proteoglycan molecule consists of a central protein core to which many sulfate glycosaminoglycan side chains (chondroitin sulfate and keratan sulfate) are attached. A proteoglycan aggregate is composed of many such molecules attached to a long hyaluronic acid chain. Link protein stabilizes this interaction. |
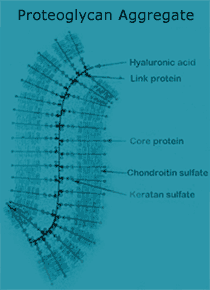 |
 |
Cell Biology of Human Chondrocytes Environmental factors influence chondrocyte morphology and the matrix that they produce. Normal adult articular cartilage consist of round chondrocytes in their lacunae arranged in a columnar organization (Fig. 4). The chondrocytes are surrounded by their matrix, mainly type I collagen and proteoglycan. To culture human adult chondrocytes in vitro, the chondrocytes have to be freed from their matrix (Fig. 5). The cartilage biopsy is removed and put through an enzymatic digestion to release the cells from their matrix. The cells are then placed in a monolayer culture dish for expansion. The cells attach to the substratum of the dish, spread, and proliferate rapidly. Healthy chondrocytes grown on monolayer culture on plastic have a fibroblastic appearance and produce predominantly type I collagen (Fig. 6). This process, termed de-differentiation. is reversible. Monolayer chondrocytes will re-express type II collagen and the large aggregating proteoglycans once placed in the proper environment, whether in suspension in vitro or in the joint. The reversibility of this process is key to the successful repair of articular cartilage with cultured autologous chondrocytes. |
||
Figure 4. Immunohistological Section Through the Joint. Normal adult articular cartilage consist of round chondrocytes in their lacunae arranged in a columnar organization. Type II collagen (brown) surrounds the chondrocytes. |

|
Figure 5. Cultured Chondrocyte Processing and Implantation Technique. A healthy cartilage biopsy is removed and put through an enzymatic digestion. The cells are then expanded on a monolayer culture. The cells are then released from the substratum of the dish. These chondrocytes can be placed in either an in vitro suspension culture or in the joint where they will redifferentiate and re-express the cartilage phenotype. |
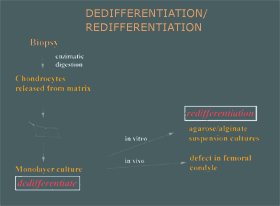 |
Figure 6. Articular Chondrocytes Grown in Monolayer Culture. Chondrocytes attached to substratum of culture dish. They differentiate and appear as dermal fibroblasts. |

|
 |
Biochemical Characteristics of Human Chondrocytes To ensure that cultured chondrocytes subjected to proliferative monolayer
culture in tissue culture flasks retain their ability to differentiate
and form a matrix that closely approximates the character of native articular
cartilage, several differentiation models can be used. Two in vitro systems
used to examine the ability of chondrocytes to re-express the differentiated
cartilage phenotype are the culturing of chondrocytes in agarose and alginate.
These are semi-solid mediums which prevent the cells from attaching to
a substratum. When chondrocytes are suspended in agarose for 3 to 4 weeks
following expansion on monolayer culture flasks, they form colonies of
cells with "halos" of matrix surrounding the cells, characteristic
of chondrocytes (Fig. 7).16 These differentiated colonies re-express cartilage
markers such as sulfated proteoglycan, as visualized by Safranin O stain
(which stains sulfated proteoglycan orange; Fig. 8). The culturing of
chondrocytes in alginate offers an advantage over culturing the cells
in agarose to examine the re-expression of the cartilage phenotype. Differentiated
chondrocyte colonies from the alginate can be recovered at different time
points and examined for their ability to produce normal biochemical and
molecular cartilage markers. To examine the Molecular Markers for Chondrocytes Gene expression during proliferation and subsequent re-differentiation
of cultured human chondrocytes is also being examined (Fig. 12). Total
mRNA is probed with specific reverse transcribed probes for the presence
of the following genes: aggrecan, type II collagen, type I collagen, and
18S rRNA. To control for RNA integrity and yield, the RNA samples are
tested for hybridization to 18S rRNA cDNA. Chondrocytes grown in monolayer
cultures express type I collagen mRNA. Following anchorage independent
culture in alginate, the chondrocytes switch their collagen gene expression
to predominantly type I collagen mRNA. All normal adult articular chondrocyte
strains examined demonstrate activation of type II collagen mRNA by |
||
Figure 7. Chondrocytes Grown in Semi-solid Agarose Media. Chondrocytes differentiate and form colonies in agarose. A halo of matrix surrounds the chondrocytes. |
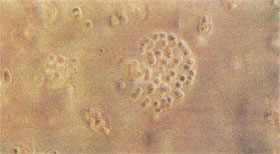
|
Figure 8. Chondrocytes Grown in Semi-solid Agarose Media and Stained with Safranin O/ Fast Green. Chondrocytes differentiate and form colonies in agarose. They re-express cartilage phenotypic markers such as sulfated proteoglycan. Safranin O stains the sulfated proteoglycan produced in the matrix by the chondrocytes orange and Fast Green stains the cells. |
|

|
||
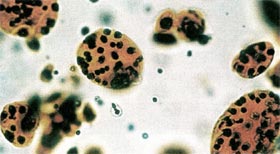
| Figure 9. Immunohistochemical
Analysis of Chondrocytes Grown in Monolayer Culture and Alginate for 4 Weeks.
Chondrocytes differentiate and form colonies in agarose. They re-express cartilage phenotypic markers such as sulfated proteoglycan. Safranin O stains the sulfated proteoglycan produced in the matrix by the chondrocytes orange and Fast Green stains the cells. |
 |
||
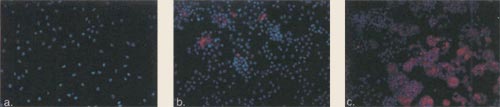
| Figure 10. Immunofluorescence
of Chondrocytes Grown in Alginate for 2, 4, and 6 Weeks. Cultured human chondrocytes were seeded into suspension culture using alginate. Following 2 weeks (a), 4 weeks (b), and 6 weeks (c) in the alginate the differentiated colonies were recovered and analyzed. Texas red conjugated type II collagen antibody (red color) and the nucleus of the cell using Hoechst dye (blue color) were analyzed at the different time points. Immunohistochemical analysis revealed that type II collagen was turned on by 2 weeks, with additional increase in colony size and further deposition of type II collagen by 4 and 6 weeks. |
|
||
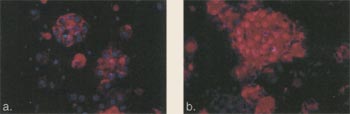
| Figure 11. Immunofluorescence
of Chondrocytes Grown in Alginate for 2 and 6 Weeks. Cultured human chondrocytes were seeded into suspension culture using alginate. Following 2 weeks (a) and 6 weeks (b) in the alginate, the differentiatied colonies were recovered and analyzed. Texas red conjugated chondroitin sulfate antibody (red color) and the nucleus of the cell using Hoechst dye (blue color) were analyzed at the different time points. Immunohistochemical analysis revealed that chondroitin sulfate was turned on by 2 weeks, with additional increase in colony size and further production of chondroitin sulfate by 6 weeks. |
Figure 12. Time course of Chondrocytes Differentiation in Alginate. Total RNA, isolated from monolayer cultured chondrocytes and alginate cultured chondrocytes at timed intervals, was probed with specific transcribed probes for the presence of the following genes: aggrecan, type II collagen, type I collagen, 18S rRNA. Chondrocytes grown in monolayer cultures express type I collagen RNA. Following anchorage independent culture in alginate the chondrocytes switch their expression to predominately type II collagen RNA. Another specific marker of hyaline cartilage, aggrecan was not turned off in monolayer culture, and was significantly enhanced by suspension culture. |
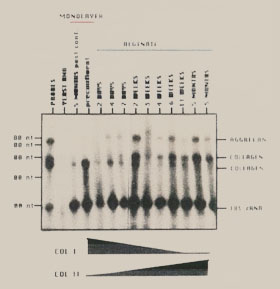 |
 |
Cultures were also examined for expression of
other collagen genes: type X collagen and type IX collagen (Fig. 13). Chondrocytes
grown in monolayer cultures express type I collagen mRNA, as do dermal fibroblasts.
Following anchorage-independent culture in alginate, the chondrocytes switch
their gene expression to predominantly type II collagen, with some type
IX collagen (a minor component of hyaline cartilage). In contrast, type
X collagen, an indicator of chondrocyte hypertrophy and eventual bone formation,
was not turned on by adult articular chondrocytes grown in monolayer or
anchorage-independent cultures out to 5 months. This demonstrates that the
adult articular chondrocytes are committed cells that differentiate into adult articular cartilage when implanted into chondral defects and are not capable of differentiating further into bone. In contrast, cells derived from periosteum, perichondrium, or mesenchyme express type X collagen and have been shown to form bone upon implantation into chondral defects.17-19 Cultured Autologous Chondrocytes to Repair Chondral
Defects in a Canine This study was designed to evaluate the role of cultured autologous chondrocytes
in the repair of full-thickness articular cartilage defects in a weight-bearing
animal model, the adult canine. |
||
Figure 13. Collagen expression during the in vitro differentiation of articular chondrocytes. Total RNA, isolated from fibroblasts, monolayer cultured chondrocytes and alginate cultured chondrocytes at timed intervals, was probed with specific transcribed probes for the presence of the following genes: type X collagen, type II collagen, type I collagen, and type IX collagen. Chondrocytes grown in monolayer cultures express type I collagen RNA, as do dermal fibroblasts. Following anchorage independent culture in alginate the chondrocytes switch their expression to predominately type II collagen RNA, with some type IX collagen (a minor component of hyaline cartilage). Type X collagen, an indicator of chondrocyte hypertrophy and eventual bone formation, is never turned on by adult articular chondrocytes. |
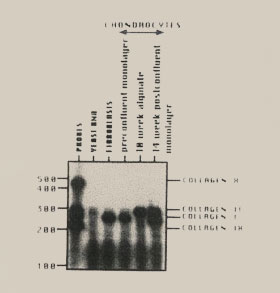 |
Figure 14. Macroscopic view of defect created in the canine model. Two cylindrical focal cartilage defects (4 mm diameter) were created in the femoral condyle in the canine. Articular cartilage was removed down to but not through the subchondral bone plate. |
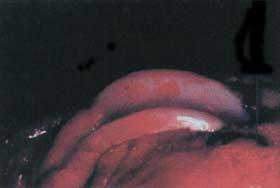 |
|||
|
||
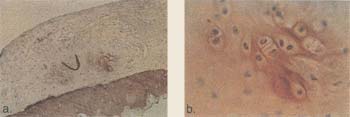
| Figure 15. Immunohistochemical
section of type II collagen of the defect treated with chondrocytes in the
canine model at 6 weeks. Autologous chondrocytes were implanted into focal defects created in the canine model. Animals were sacrificed at 6 weeks and analyzed for type II collagen. Type II collagen is a specific phenotypic marker for articular cartilage. a) Differentiating chondrocytes expressing type II collagen with developing lacunae are apparent in chondrocyte-treated defects. b) Close-up of nest. |

|
||
| Figure 16. Chondrocytes
genetically tagged with b-galactosidase reporter gene in the canine model
at 6 weeks. To ensure that the chondrocytes that were implanted into the defect are facilitating the repair autologous chondrocytes were transfected in vitro with the b-galactosidase gene and implanted into defects created in the canine model. Animal were sacrificed at 6 weeks and analyzed by histology and immunohistochemistry. a) Cross-section of defect treated with labelled cells and covered with periosteum. Chondrocytes labelled with the gene will turn blue when reacted with the x-ga substrate. b) Immunohistochemical section of the defect containing the b-galactosidase treated chondrocytes and stained with type II collagen (brown). Note: type II collagen matrix surrounds the b-galactosidase treated cells. |
|
||
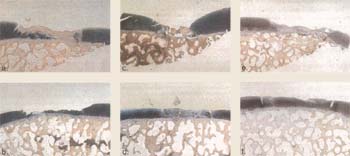
| Figure 17. Histological
sections of defects stained with hematoxylin and eosin in the canine model
at 6 months (26 weeks). Defects were created in the femoral condyles in the canine model. Defects were either treated with no cells in the left knee (a, c, e) or treated with cultured autologous chondrocytes in the right knee (b, d, f) in three separate animals (ab, cd, ef). Animals were sacrificed at 6 months and defects were analyzed by Hematoxylin and Eosin histology. Results revealed that there was hyaline-like cartilaginous fill in both treated and untreated defects. There was a general trend toward more hyaline, articular cartilage fill in cell treated defects (b, d, f). |
|
||
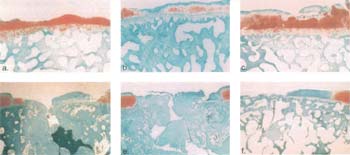
| Figure 18. Histological
sections of defects stained by safranin O in the canine model at 1 year
(52 weeks). Focal cartilage defects were surgically created in the femoral condyles of the canine model. Animals were separated into three treatment groups: untreated defects (a, d); untreated defects covered with periosteum (b, e); or defects implanted with cultured autologous chondrocytes and covered with periosteum (c, f). Animals were sacrificed at 1 year and defects analyzed for Safranin O. At one year, the trend seen at 6 months was not apparent. Levels of fibrous tissue, articular cartilage, and subchondral bone penetration in all treatment types were virtually identical. Significant levels of spontaneous regeneration of hyaline-like cartilage were noted in those defects having an uncompromised subchondral bone plate. |
 |
Conclusions Acknowledgments Genzyme tissue repair Genzyme Genzyme B.V.
|
||
|
|||||||